If you work in healthcare revenue cycle management, you know the drill: “If it wasn’t documented, it wasn’t done.” But in today’s complex regulatory landscape, simply writing down what happened isn’t enough. Clinical documentation must now serve multiple masters—supporting patient care, justifying reimbursement, and meeting an ever-evolving list of compliance standards from the Centers for Medicare & Medicaid Services (CMS) and private payers.
Navigating the maze of medical necessity, coding specificity, and payer-specific rules can feel like trying to hit a moving target. Inaccurate or incomplete clinical documentation is the leading cause of claim denials, delayed payments, and audit failures. Whether you are a provider, a coder, or a practice manager, understanding the baseline requirements is critical for financial health and operational efficiency.
This guide provides a comprehensive, simplified checklist of the essential documentation standards you need to know to keep your claims clean and your revenue flowing.
Introduction to CMS and Private Payer Documentation
The healthcare industry is currently undergoing a massive shift toward transparency and data interoperability. Historically, documentation was primarily about liability and basic billing. Today, it is the foundation of value-based care, health equity initiatives, and automated prior authorizations.
Both CMS and private payers (like UnitedHealthcare, Aetna, and Blue Cross Blue Shield) have aligned many of their requirements, yet distinct nuances remain. The core principle, however, is universal: the medical record must tell the complete story of why a service was needed, what was done, and how the patient responded.
The stakes are high. CMS audits, such as those conducted by Recovery Audit Contractors (RACs), specifically target insufficient clinical documentation. Private payers are following suit, using sophisticated algorithms to flag claims that lack specific data points. By standardizing your approach to documentation based on federal guidelines, you create a safety net that protects your practice against scrutiny from all payer types.
Read More >> AI Clinical Workflows in Action: Real-World Examples of Human-AI Synergy
Key Requirements for Clinical Documentation
To ensure compliance and optimal reimbursement, your documentation must meet specific structural and content standards. Below is a breakdown of the critical elements that must be present in the patient’s medical record.
Written Order and Prescription Standards
At the heart of any claim for Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS), or for specific diagnostic tests, is the order.
All claims submitted to Medicare Part B require a written order or prescription from the treating practitioner. This is a non-negotiable condition for payment. The order validates that a physician or qualified non-physician practitioner (NPP) has evaluated the patient and deemed the item or service medically necessary.
Checklist for Orders:
- Treating Practitioner Identification: The order must clearly include the name and National Provider Identifier (NPI) of the treating practitioner.
- Signature: It must be signed and dated by the treating practitioner. Stamped signatures are generally not accepted; electronic signatures must meet CMS security standards.
- Consistency: The order must match the claim exactly regarding the items billed (HCPCS codes) and the diagnosis codes.
Standard Written Order (SWO) vs. Written Order Prior to Delivery (WOPD)
Understanding the distinction between an SWO and a WOPD is crucial for DMEPOS suppliers.
Standard Written Order (SWO):
An SWO must be communicated to the supplier prior to claim submission. It must contain:
- Beneficiary Name or Medicare Beneficiary Identifier (MBI).
- Order Date.
- General Description of the item (e.g., “hospital bed” or specific brand/model).
- Quantity to be dispensed (if applicable).
- Treating Practitioner Name or NPI.
- Treating Practitioner Signature.
Written Order Prior to Delivery (WOPD):
For certain high-cost or high-risk items (like Power Mobility Devices), the requirements are stricter. A specific list of items, known as the “Required List,” mandates that a completed SWO be communicated to the supplier before the item is delivered to the beneficiary.
- Timing: The WOPD must be completed within six months after the required face-to-face encounter.
- Delivery: You cannot deliver the item until this document is in hand. Delivering early can result in an automatic denial.
ICD-10 Z-Code Diagnosis Capture and Reporting
One of the most significant recent shifts in clinical documentation is the emphasis on Social Determinants of Health (SDOH). CMS and private payers are increasingly requiring data on the non-medical factors influencing a patient’s health.
This is where ICD-10 Z-codes (Z55-Z65) come into play. These codes describe socioeconomic and psychosocial circumstances, such as housing instability, food insecurity, or lack of transportation.
Why Z-Codes Matter:
- Holistic Care: They paint a fuller picture of patient complexity.
- Risk Adjustment: They can influence risk-adjustment scores, which are vital for Medicare Advantage and value-based contracts.
- Resource Allocation: They justify the need for additional support services, such as case management or social work.
Documentation Tips for Z-Codes:
- Source of Information: Unlike medical diagnoses, coding guidelines allow SDOH data to be collected from non-physician staff (like social workers or community health workers) and even patient self-reporting, provided it is signed off by the clinician and incorporated into the medical record.
- Frequency: Assign Z-codes as often as the conditions are documented. If a patient faces housing instability at every visit, code it at every visit.
- Secondary Codes: Remember, Z-codes are generally used as secondary diagnoses, not the primary reason for the encounter.

Documentation Timeframes and Record Keeping
Timeliness is just as important as accuracy. Documentation that appears legally valid but was created weeks after the service date raises red flags for auditors.
The 7-Year Rule
CMS requires that documentation be maintained in the supplier’s or provider’s files for seven years from the date of service (DOS). This includes the SWO, proof of delivery, and medical records supporting medical necessity. If you are audited, you must be able to retrieve these records promptly.
Contemporaneous Records
The “golden rule” of clinical documentation is that it should be contemporaneous. This means notes should be created at the time of service or shortly thereafter.
- Continued Medical Need: For ongoing services (like rental equipment or recurring supplies), the medical record must show timely documentation—typically defined as a record generated within the preceding 12 months—demonstrating that the item remains reasonable and necessary.
- Refills: For supply refills, you cannot simply put a patient on “autopilot.” The record must document an affirmative response from the patient (or caregiver) requesting the refill before shipment occurs.
Proof of Delivery (POD)
For DMEPOS, the Date of Service is generally the Date of Delivery. You must maintain a Proof of Delivery document that includes:
- Beneficiary’s name.
- Delivery address.
- Detailed description of items.
- Quantity delivered.
- Date delivered.
- Beneficiary (or designee) signature.
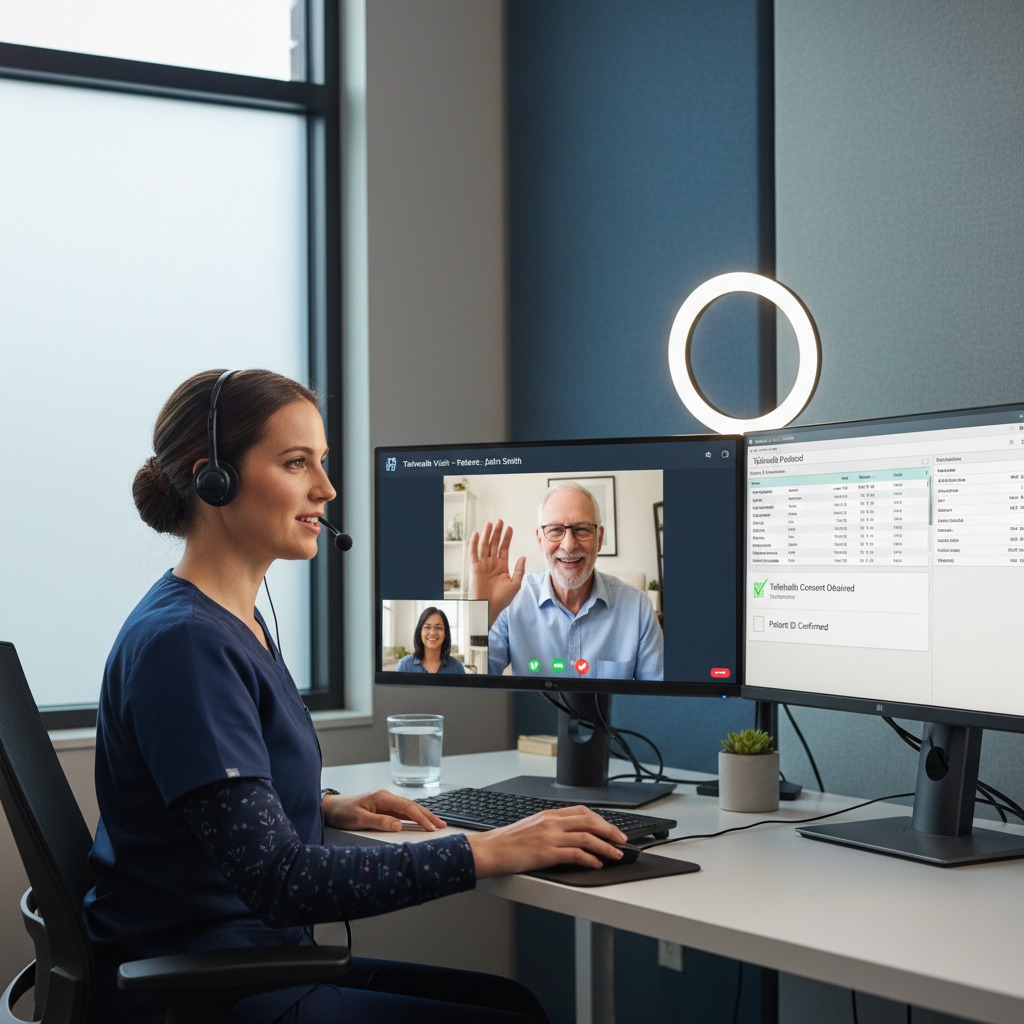
Telehealth Documentation Guidelines
Telehealth is no longer a temporary measure; it is a permanent fixture of the healthcare ecosystem. Consequently, CMS and private payers have solidified their documentation expectations for virtual visits.
While the clinical content of a telehealth note should mirror an in-person visit (history, assessment, plan), there are specific administrative additions required to ensure payment.
Telehealth Checklist:
- Consent: Document that the patient provided verbal or written consent to receive services via telehealth. Note whether this consent was obtained at the start of the visit.
- Modality: Explicitly state how the visit was conducted. Was it a real-time audio-video synchronization? Was it audio-only (if permitted for the specific service)?
- Location: Document the location of the provider and the location of the patient. This is critical for determining “originating site” compliance and potential geographical payment adjustments.
- Time-Based Billing: Since many telehealth services are billed based on time, accurately record the start and stop times or the total duration of the encounter.
- Platform Security: While not always required in the medical note itself, maintaining internal logs that the platform used was HIPAA-compliant is a best practice.
For mental health providers specifically, note that while audio-only visits are permitted in certain contexts, the medical necessity for excluding video must be documented (e.g., patient lacks broadband access or refused video).
CMS and Private Payers Interoperability and Prior Authorization API Requirements
The days of faxing prior authorization requests are numbered. CMS has finalized rules aimed at streamlining the prior authorization process through the use of Application Programming Interfaces (APIs). This initiative is designed to reduce the burden on providers and speed up access to care for patients.
The Impact on Documentation:
The new “Prior Authorization API” allows provider practice management systems to query a payer’s system directly.
- Automated Requirements: The API will tell the provider exactly what clinical documentation is required for a specific item or service.
- Digital Submission: It allows providers to compile and submit this documentation electronically from within their workflow.
- Transparency: Payers must provide specific reasons for denials, which helps providers understand exactly what documentation was missing or insufficient.
Timeline for Payers:
By 2026 and 2027, Medicare Advantage, Medicaid, CHIP, and Qualified Health Plan payers are required to implement these APIs. This means providers must prepare their internal systems to interface with these new digital pathways. The goal is to move toward a “documentation by exception” model, where routine approvals are automated, and only complex cases require manual review.
Social Determinants of Health (SDOH)
As mentioned in the Z-code section, SDOH is moving from a “nice-to-have” to a “must-have” in clinical documentation. CMS’s strategic plan explicitly links health equity to data collection.
The Business Case for SDOH Documentation:
- Quality Reporting: SDOH screening is becoming a quality measure in various reporting programs (like MIPS and ACO REACH). Failure to document screenings can lower quality scores and subsequent reimbursement.
- Resource Connection: Private payers are increasingly launching programs to assist members with food and housing. They cannot offer these resources if the provider does not document the need.
- Future Reimbursement: CMS has introduced G-codes (like G0136) for SDOH risk assessments. To bill these codes, the medical record must show a standardized, evidence-based assessment was performed.
Implementation Strategy:
Ensure your Electronic Health Record (EHR) is configured to capture these data points as structured data, not just free text in a note. This allows for easier reporting and API integration.
Conclusion: Ensuring Compliance for Optimal Reimbursement
The landscape of clinical documentation is shifting—from volume to value, and from paper to pixels. While the administrative demands may feel heavy, these requirements exist to protect patient safety and ensure fiscal integrity. At Care Medicus, we believe the organizations that succeed will be those that stop viewing documentation as a burden and start treating it as a strategic asset.
The path forward is clear. By securing valid orders, capturing Z-codes, maintaining contemporaneous records, adhering to telehealth documentation standards, and preparing for API-driven interoperability, healthcare organizations can insulate their revenue streams from audits and denials. Effective documentation does more than meet compliance—it creates stability, transparency, and confidence across the entire revenue cycle.
Now is the time to take action. Audit your workflows by reviewing recent charts against retention, SWO, and Z-code requirements. Ensure your EHR is prepared for new G-codes related to social determinants of health and future API integrations. Most importantly, educate both clinical and administrative teams on why these data points matter and how they directly impact reimbursement and risk.
Documentation is the bridge between the care you deliver and the revenue you deserve. Strengthen that bridge today, and you protect your practice’s future for tomorrow. With deep expertise in compliant documentation and revenue cycle strategy, Care Medicus helps healthcare organizations turn documentation into a source of resilience, accuracy, and long-term success.





Leave a Reply